|
|
 |
|
|
|
 Auro Laboratories is a manufacturer of Active Pharmaceutical Ingredients (API's),
Intermediates and Generic Formulations. Auro Laboratories was
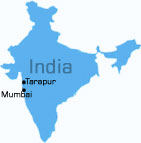
founded and incorporated in 1992 at Mumbai, with a manufacturing facility located at
Tarapur, 120 Km from Mumbai.
Since inception we have focused on providing quality pharmaceuticals while maintaining
high manufacturing standards. Auro Laboratories is a manufacturer of Active Pharmaceutical Ingredients (API's),
Intermediates and Generic Formulations. Auro Laboratories was
founded and incorporated in 1992 at Mumbai, with a manufacturing facility located at
Tarapur, 120 Km from Mumbai.
Since inception we have focused on providing quality pharmaceuticals while maintaining
high manufacturing standards.
Our primary focus has been on Anti-Diabetic Drugs, which is estimated to be one of the fastest
growing therapeutic segments. Currently, we manufacture Metformin Hydrochloride and
Glibenclamide and are on the anvil of introducing others within this segment. Additionally, we
also undertake Toll/Custom Manufacturing for API's and Intermediates on contract basis. The
manufacturing facility operates are per cGMP norms and has an installed capacity in excess of
500 Tonnes per annum. |
|
| Auro laboratories has made its presence felt in international
markets with a bulk of our production being exported to South East Asia, Middle East and
several European Countries. Some of the countries we export to are Egypt, Germany, Malaysia, Singapore, South Africa, Brazil, Spain and
United Kingdom. |
|
| We believe in rigorous implementation
our Quality Management Systems, which ensure the manufacturing
of quality products with due environmental concerns. This
has enabled us to successfully achieve ISO
9001:2000 and WHO - cGMP
certifications. We have received CERTIFICATE OF SUITABILITY
(COS) from the European Directorate of Quality Medicine
(EDQM) in September 2006 vide Certificate No.R0-CEP 2005-123-
Rev 00. |
|
 |
|
| Since inception, we have built a solid foundation & achieved steady growth over the years.
|
|
| We believe this world help us propel to further heights in the future. |
|
 |
|
|
|
|
|
-
Auro
Labs started its operations with
Sodium Citrate and Potassium Iodide.
-
We
then developed specific expertise
in the antibacterial drug, Trimethoprim
|
|
|
|
-
We
went for backward integration
and started producing 3,4,5 Trimethoxy
Benzaldehyde, the basic raw material
for Trimethoprim
-
IPO
of shares in 1996, currently listed
on the Bombay Stock Exchange (BSE)
|
|
 |
-
Achieved
ISO 9001:2000 Certification
|
|
 |
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
Copyright © Auro Laboratories Limited, 2005. All rights reserved. |
|